Improvement of seed production by SISP-B (Semi Intensive Seed Production with Balanidae)
Improvement of seed production by SISP-B (Semi Intensive Seed Production with Balanidae)
Abstract
Wild captured cabrilla común (Paralabrax humeralis) spawn 19 million
eggs and 9 million larvae hatch-out from November 2018 to April 2019. The
spawning occurred water temperature 18 to 20 °C mainly. Several SISP (Semi Intensive Seed Production)were operated
and improved the method. SISP method is different from intensive method as SISP
feed natural growing wild copepod instead of rotifer and Artemia that create
intensively. Thus SISP not only require several tanks and equipment (pump,
filter, UV etc.) but also high level technique and experience. SISP makes
anybody produce premium juvenile easily without large investment. Remarkably we
found that the larvae eat picoroco nauplius (Austromegabalanus psittacus) which nauplius grows bigger and longer
than copepod and blue mussel. Also produce picoroco nauplius easier than copepod
that collect in coast and just hang in the SISP tank. We modified SISP-C to
SISP-B and producing some cabrilla juveniles.
Introduction
Wild captured cabrilla común (Paralabrax humeralis) spawn 19 million
eggs and 9 million larvae hatch-out from November 2018 to April 2019. The
spawning occurred water temperature 18 to 20 °C mainly. Several SISP (Semi Intensive Seed Production)were operated
and improved the method. SISP method is different from intensive method as SISP
feed natural growing wild copepod instead of rotifer and Artemia that create
intensively. Thus SISP not only require several tanks and equipment (pump, filter,
UV etc.) but also high level technique and experience. SISP makes anybody produce
premium juvenile easily without large investment.
Remarkably we found that the larvae eat picoroco nauplius (Austromegabalanus psittacus)
which nauplius grows bigger and longer than
copepod and blue mussel. Also produce picoroco
nauplius easier than copepod that collect in coast and just hang in the SISP
tank. We modified SISP-C to SISP-B and producing some cabrilla juveniles.
Material and Method
The SISP operated cuboid tank (3 x 3
x 2.4 m, 20 m3) and round tank (6.4Ø m x 1.2 m, 35 m3),
large volume tank minimizes fluctuation of water temperature and improves
phytoplankton propagation. At beginning, supplied sea water through 1 m/m
bag-net, gave aeration and fertilizer, potassium nitrate 3 g/m3 and
triple superphosphate 0.5 g/m3. Supplied water 10 % a day
continually and control propagation of phytoplankton. We maintained pH 8.2 to
8.6 with water supply, fertilizer application and sheading surface. After a
week phytoplankton (Chaetoceros spp.)
grew and water color turned to brown (Figure).
Selected premium larvae, floating egg rate and hatch-out rate show over
90 %, high survival rate until DPH2 (Days Post Hatch-out), and stocked in the
tank at density of 2 and 3 larvae/L.
We took one litter of water and
filtered by 63 µm mesh (Figure). Nauplius
of copepod and picoroco, copepod adult and Polychaeta larva were counted daily.
Fed grinded Choro gonad (Mytilus chilensis, 1 million
eggs/g, Figure) during DPH3 to 13. Also we fed Balanidae nauplius that
installed inside Nauplius Supplier (NAS) or keep in pail, 1 Kg/10 m3,
and supply water continually (Figure).
Result
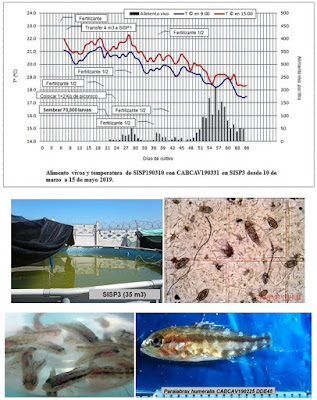
The culture SISP190310 started with
round tank (SISP3, 35 m3, plastic swimming pool) on March 10 and
stocked 70,000 of hatch-out larva on March 31. We fed choro eggs and installed NAS,
and live feeds propagated over 200 per liter. We harvested 45 larvae, LT 22mm
and BW 0.14g, on DPH 45, survival rate 0.06 %, on May 15. The reason of low
survival is mas-mortality just after stocking possibly. Because this batch was not
confirmed survival from DPH 0 to 2, sometime mortality occurs before start
eating feeds due to larva handling and quality of eggs.
Discussion
Based on several operations of SISP,
we recommend following production model of SISP-B.
1.
Introduce
initial water through 1 m/m mesh bag-net, covered by black Rachel (85 %), and
apply fertilizer, potassium nitrate 3 g/m3 and triple superphosphate
0.5 g/m3, with gentle aeration. Water supply 5 % daily continually.
2.
Wait
few days to propagate Chaetoceros
spp. and water color turn to brown. Possibly some nauplius and adult of copepod
are appear.
3.
Stocking
hatch-out larva at density of 2 to 5 larvae/m3 of SISP tank volume
to Monitoring tank (conical 500L) which floating inside SISP tank. Observe larva
survival until DPH2 and release to SISP tank if survival rate show over 80 %.
4.
Feeding
blue mussel gonad at quantity of 1 g/ m3 from DPH3 to 13 daily. Also
install Nauplius Supplier (NAS) with barnacles 2 Kg/10 m3 from DPH5
to 60. If reduce microalgae in SISP tank, supply water from phytoplankton tank,
swimming pool (6.5Øx1.2m), by diaphragm pump or gravity. Feed artificial feeds
for larva to juvenile, e.g. Otohime C1 (650 µm) and C2 (1.1 mm) from DPH25
depend on size of juvenile and availability of live feeds.
5.
Harvest
totally on DPH45 to 60 when juvenile reach to 3 cm and keep in nursery tank, 2
to 4 m3 round tank and rearing intensively, feed only artificial
feeds and high water exchange with water current.








Comments
Post a Comment